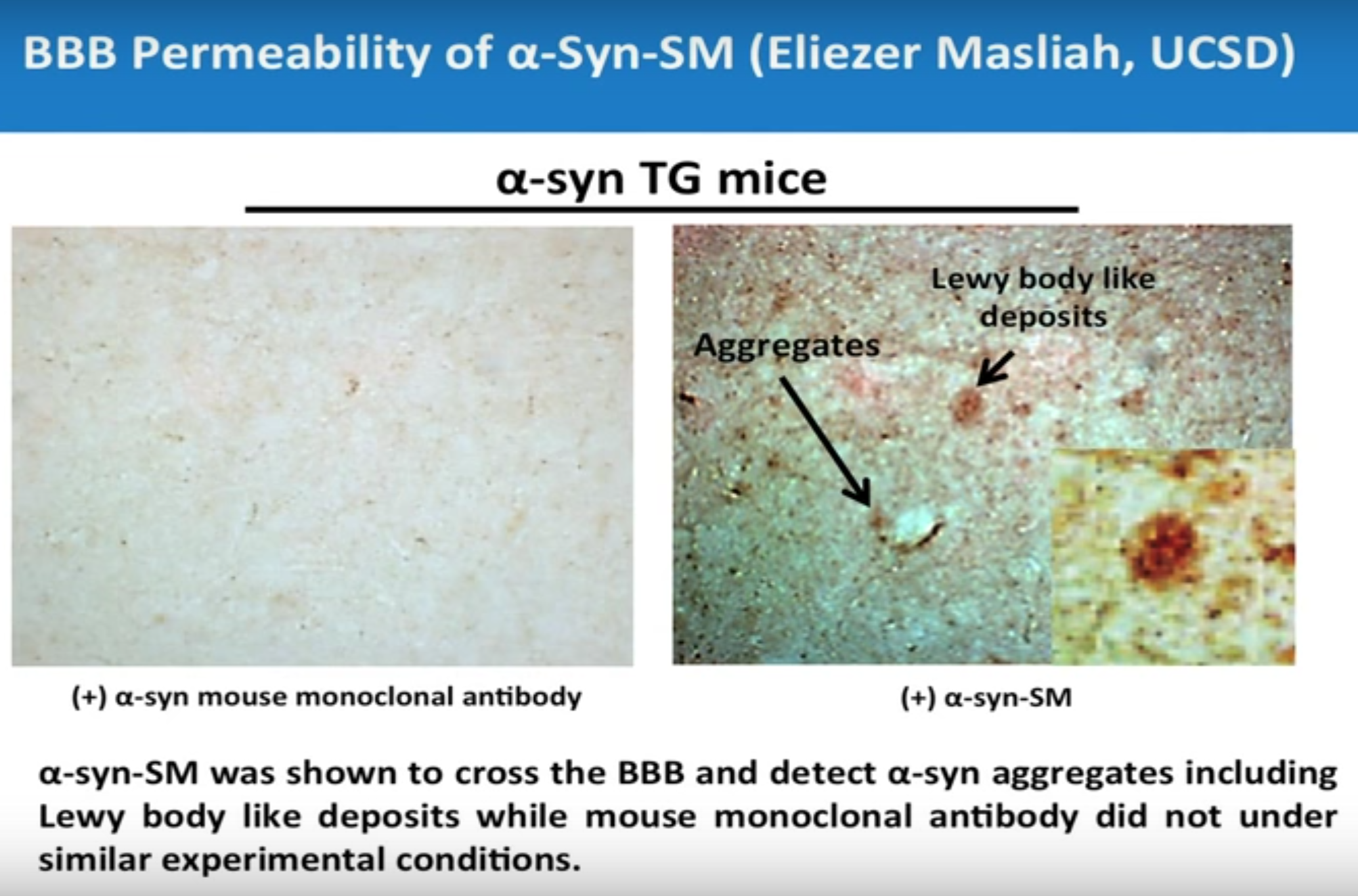
L A JOLLA, Calif. – May 11, 2014 – On May 11, 2014, ICB International, Inc., (“ICBI”), announced confirmation of third-party validation provided by Dr. Eliezer Masliah of the University of California, San Diego. Dr. Eliezer Masliah has shown, upon being provided the necessary materials by the ICBI team, that the amyloid-beta-SM molecule and the α-Synuclein-SM traffic the blood-brain barrier, breach the blood-brain barrier and reach their target, whereas the amyloid-beta mouse monoclonal antibody and the α-Synuclein mouse monoclonal antibody do not.
Validation of ICBI’s blood-brain barrier permeability and target specificity by Dr. E. Masliah points to the clinical potential of the alpha-synuclein Smart Molecule. The experiments conducted by both parties proves ICBI’s ability to penetrate the blood brain barrier in an alpha-synuclein transgenic moue and also shows how it is specifically bound to both Lewy body-like deposits and α-syn aggregates.
“These recent discoveries place ICBI among the
potential world leaders in efforts to develop
diagnostics and therapies for brain disorders,”
says founder, Dr. Ram Bhatt.
ABOUT THE COMPANY: ICBI is a privately-owned La Jolla, CA based biotech Company engaged in developing proprietary modular platform technology, SMART Molecules, for diagnosing and treating diseases of the central nervous system and cancers of all types. Website: www.icbii.com apotekerendk.com.
FORWARD-LOOKING STATEMENTS
This press release may contain forward-looking statements, including ICBI’s development of SMART Molecules as SPECT/PET ligands for Parkinson’s disease. Each of these statements may involve risks and uncertainties. Actual results may differ materially from those, expressed or implied.
Media Contact: Ram Bhatt, ICB International, Inc., 858-455-9880, rbhatt@icbii.com.